Create a free profile to get unlimited access to exclusive videos, sweepstakes, and more!
How to make strontium: Collide two neutron stars and stay *way* back

On 17 August, 2017, astronomers detected an event they had long suspected could happen but had never seen before: the catastrophic merger of two neutron stars. Born billions of years before and orbiting each other ever since as a binary system, they had slowly spiraled closer together until they finally crashed into one another. The force of the merger literally shook the fabric of space-time — they generated gravitational waves that were the first sign of the event — and collapsed them into a black hole.
It also generated a massive explosion that was far brighter than a regular nova, but something less than a supernova; what astronomers call a kilonova. This had been long predicted but never seen. The huge burst of light fades rapidly, so catching one when it's still bright is incredibly difficult.
This explosion also seemed to solve a major mystery in astronomy: The creation of r-process elements. We see lots of elements like barium, gold, silver, platinum, and so on in the Earth, and we know they must be made in some sort of hugely explosive event, but how, exactly? To make them you need to bombard other atoms with lots and lots (and lots) of neutrons, and do so rapidly (which is why it's the r-process) and under high pressure and temperature. That's possible in a supernova, but unlikely. A far better environment is the merger and explosion of two neutron stars — neutrons aren't a problem with them, as you can guess from the name — but that had never been seen.
Until August 2017. The light from the merger and subsequent kilonova was broadly consistent with what you'd expect to be emitted from r-process elements, and this was considered a successful confirmation of the hypothesis. And that's still true… but the observations didn't seem to show any features from a specific r-process element.
New research, however, has changed that. A team of astronomers re-reviewed the early observations of the kilonova, and in a paper just published make the case that they see the fingerprints of the element strontium in them.
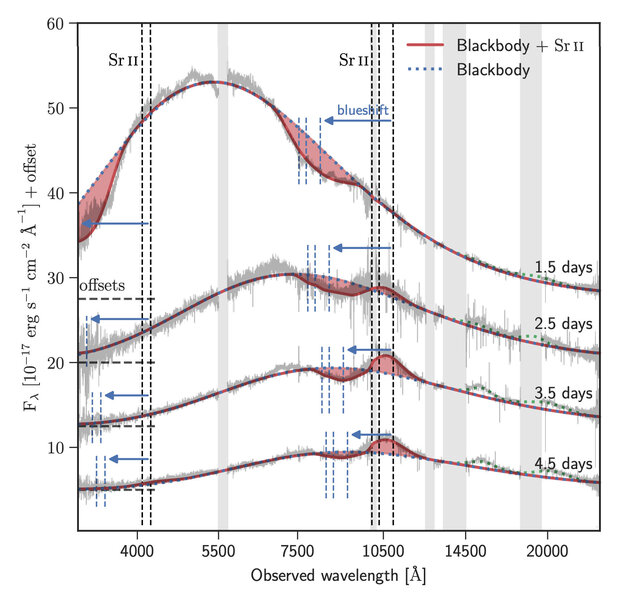
Their reasoning is a bit subtle, and depends on the spectra taken literally days after the event. A spectrum is taken by breaking up the light from an object into lots of individual colors, like a rainbow but far more detailed. Different elements absorb light at different wavelengths (colors), so if you see a dip in the light at a specific wavelength, you can in theory identify the element or elements involved.
That gets harder in explosions from stellar mergers! The fireball itself emits a smooth distribution of light (for historical reasons called a blackbody curve), and elements in it will absorb some of that light. But that material is screaming outward at a significant fraction of the speed of light, and that changes its color due to the Doppler shift. Worse, some stuff is headed toward at you one speed, and other stuff is headed toward you slightly slower or faster, smearing out its profile. Instead of a sharp line of absorption, you get a broad dip.
… but that's just what they saw in the kilonova's spectrum! A broad dip in the otherwise pretty smooth spectrum. The dip is centered at about .81 microns (just a bit outside what the human eye can see at the red end of the spectrum). Assuming this material is headed toward us at about 0.2 times the speed of light (!!), the only element consistent with this is strontium. It absorbs light at about 1.05 microns, which at that speed blueshifts to what is seen for the dip.
That's interesting, but not conclusive… but we're not done. Over time, the spectrum of the kilonova changed. It faded, of course. It also faded faster at the blue end then the red end, which is also expected. But the dip changed too. It changed from being a single dip, to showing this weird sine wave shape: a dip on the left (on the blue end) but a bump on the right (the red end).
I know that doesn't sound like a big deal, but that's critical! This is what's called a P Cygni profile, after the star of that name. When you get an expanding shell of gas, as it gets bigger it gets less dense. That allows you to see deeper into the gas, where it's hotter. When a gas is hot it emits light, so you get a bump in the spectrum. But because it's deeper in it's not moving as rapidly toward you, so it's less blueshifted. That puts the bump on the red end of the dip. The gas that's moving toward you rapidly is still on the outside of the shell, and still absorbing light, so you see a dip at the blue end of the feature.
That's precisely what's seen in the kilonova spectrum after a few days. It takes time to expand and for the shell to get thinner so you can see deeper into it, so this P Cygni profile doesn't appear until after a few days. And all this, once again, is consistent with it being from strontium. No other element the scientists looked at matched what was seen in the spectra.
It's likely many other r-process elements were made, but they don't show up in the spectra because they emit light at wavelengths outside what was measured (for example, very blue light close to being ultraviolet). As time goes on, and astronomers see more kilonovae, we'll be able to see spectra in the UV and the infrared, and that will greatly help IDing the elements involved.
This is very cool! The heavy elements you see all around you — iron, calcium, nickel, and more — were created in explosions like supernovae. Some, like aluminum, were created in giant stars and blown out into space in stellar winds. But for a whole slew of elements we weren't sure where they came from, or more accurately had no evidence of it. That's now changed: This work shows direct evidence of them. Got any gold? Silver? Iodine? It's very likely that came from two crashing neutron stars. How about that?
Sure, this research only seems to indicate finding one such element — strontium, of all things — but something has to be first. And now that we know how and where to look, it's only a matter of time before two more neutron stars somewhere out in the Universe have their own final, deadly embrace, and we catch their explosive light on the fly, dissect it, and see so many of the other materials that up until them have had mysterious origins.
Science is about finding things out. For this, we're only at step one. There are plenty more steps to go.