Create a free profile to get unlimited access to exclusive videos, sweepstakes, and more!
When the first stars in the Universe exploded, they *really* exploded

It’s weird how things tie together in astronomy sometimes. By studying a nearby star, astronomers have been able to determine that the very first (and now long gone) stars in the Universe exploded asymmetrically, sending out ridiculously powerful narrowly focused beams of matter moving at very nearly the speed of light!
How was this sorcery accomplished?
First, let’s take a step back — about 13.4 billion years or so.
When the Universe was very young, the only elements in it were hydrogen, helium, and a little bit of lithium. This was all in the form of gas strewn across space.
Over time, a few hundred million years after the Big Bang, this gas started to coalesce and form the very first stars. These stars were essentially all hydrogen and helium, because there was no iron, carbon, oxygen or anything else in Universe yet. In truth, this fact dominates so much of astronomy that astronomers lump all elements heavier than hydrogen and helium into a catch-all term: metals. I know, I wish they had used a different word, but we’re stuck with it now. Oxygen may not seem like a metal, but to an astronomer it is. They just use the word differently than normal people do.
Anyway, these heavier metals change the way these stars form and live their lives; lacking the heavier elements inside the first generation of stars were able to grow huge*, hundreds of times the mass of the Sun, maybe even up to a thousand times the Sun’s mass!
Like stars today, these first generation stars fused hydrogen into helium, and then helium into carbon, and so on, creating metals in their core. These stars burned through their nuclear fuel extremely rapidly, and exploded in less than a million years. The expanding supernova debris, enriched with these heavy elements, then slammed into gas around the stars, seeding them with carbon, nitrogen, and other elements. Stars born from that gas then started off their lives metal-enriched.
Some of these second generation stars exploded, further increasing the amount of heavy elements in the Universe. Those were the massive stars, but some stars born at this time were lower mass, more like the Sun. Lower mass stars user their fuel in a more miserly fashion, living for billions of years. Even tens of billions… which means they’re still around now. Today. After 13+ billion years, some of these ancient stars still exist.
To find them, astronomers search for stars with extremely low abundances of heavy elements. It’s essentially impossible to make stars like this today, so if you find one you know it must be ancient.
Well. About 3,700 light years away from Earth is a faint, relatively non-descript star called HE 1327−2326. It’s a bit like the Sun, though slightly less massive. It’s also nearing the end of its long, long life, starting its expansion into a red giant. Right now it’s what’s called a subgiant.
It was discovered in the Hamburg/ESO survey in 2005 by astronomers looking for very old stars. It is incredibly low in some metals; the amount of iron in the star is only 0.000006 times the Sun’s!
Here’s where things get fun. Zinc is an important metal to astronomers studying ancient stars. That first generation of super-massive stars probably made lots of zinc in their cores, but it was deep down, near the star’s center. Models of how these stars explode show that when the star did go supernova, that layer of star didn’t get blasted out; instead, it collapsed down with the rest of the star’s core to form a black hole.
When they examined HE 1327−2326, they found that it did have a teeny amount of zinc in it, about 0.00004 times the Sun’s amount. However, that’s still a lot more than you’d expect for such an old star! Where did this zinc come from?
Here’s the sneaky bit. Models of supernovae from that first generation of superstars made a big assumption: That the stars exploded symmetrically; that is, the debris flew away as an expanding sphere. One reason for this is that this is way easier to model in a computer; you only have to worry about one dimension (the radial direction, away from the center; if the expansion is perfectly spherical it can be described using only that).
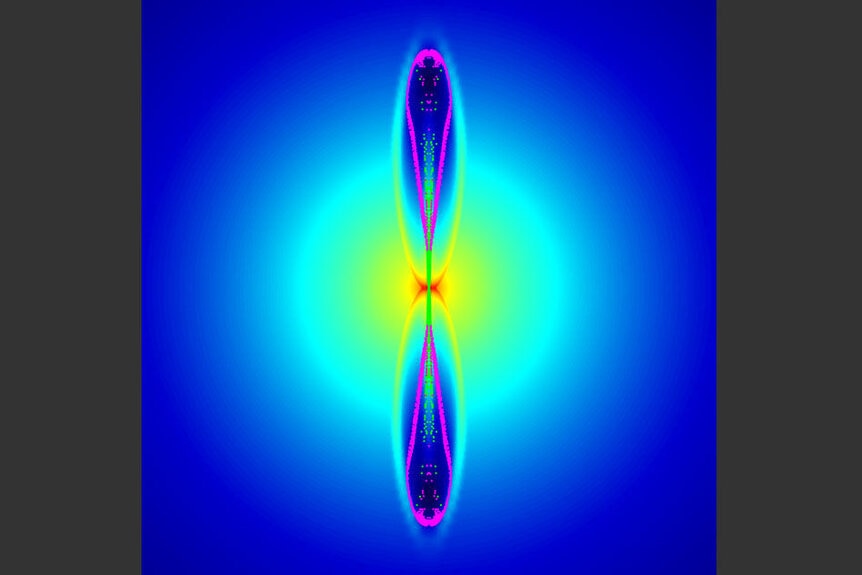
But what if the explosion isn’t spherical? We know that some stars explode off-center, for example. And even more critically, some stars rotate rapidly, and when their core collapses that rotation increases hugely (think of an ice skater spinning with their arms extended, then bringing their arms close in; their spin accelerates). This increase in spin causes the material falling into the core to flatten out into a disk, and when that happens it can focus the explosion, driving two tightly focused beams of matter and energy up and down, screaming out from the core. This plows through the upper layers of the star, tearing it apart, and even then the beams keep going; death rays marching across the Universe.
Nowadays we call such an event a gamma-ray burst, and they are one of the most powerful and terrifying things the cosmos can produce.
But they can also save the day! Because they start deep down inside the star, they can push material out that would otherwise be locked up near the core… including zinc. And sure enough, models that include beaming can produce the right amount to account for what’s seen in HE 1327−2326.
Hurray! And that’s how an ancient anemic (huh, literally) star close to the Sun revealed how even more ancient massive stars exploded near the dawn of the Universe itself. Neat.
But wait! There’s one more thing!
These beams of matter blasting away from the first stars were incredibly powerful, and difficult to stop. Moreover, the Universe was smaller then, so things were closer together. It turns out these stars could’ve seeded gas clouds pretty far away from them, peppering them with heavy metals. Now, billions of years of Universal expansion later, these clouds could be in different galaxies, but still seeded by the same star.
I would love to see someone calculate the odds of having an atom or two of from one of these long-dead and cosmically distant stars in our bodies. It’s cool enough we have atoms from supernovae and kilonovae in us; this would be even cooler.
As Carl Sagan said, we are star stuff. But it turns out, perhaps more accurately, that we are stars’ stuff.
*There are complex physical reasons for this, but some metals inside a star trap the energy produced, which makes the star hotter. If a star gets too massive it gets so hot it’ll tear itself apart; the most massive stars in the Universe now top out at a 100 or so times the mass of the Sun, and are incredibly rare.