Create a free profile to get unlimited access to exclusive videos, sweepstakes, and more!
New musical instrument translates sounds of every element on the periodic table
Give it up for the Acoustic Chemical Orchestra!

The universe is alive with the sound of music. Humans will find music almost anywhere. We find it in the songs of birds or the long-distance communications of whales. We hear music in the chirping of crickets and the purring of our cats. Even bacteria make sounds! We find animals so musical, in fact, that we imagine them holding singing competitions to save failing theaters (the premise of the 2016 animated film Sing). Our capacity for seeking out melodies isn’t limited to the animal kingdom. We hear rhythm in the tinkling of water dripping from a melting icicle and harmonies in the pulsating of black holes. We find music even in the elemental building blocks of the universe around us.
Using a technique known as data sonification, which translates information of practically any kind into soundwaves, W. Walker Smith crafted a new musical instrument from the translated sounds of every element on the periodic table. This acoustic view of chemistry allows for the creation of new kinds of music in addition to a new tool for understanding the nature of the universe. Smith presented his work at the 2023 spring meeting of the American Chemical Society (ACS).
RELATED: This is what a black hole sounds like
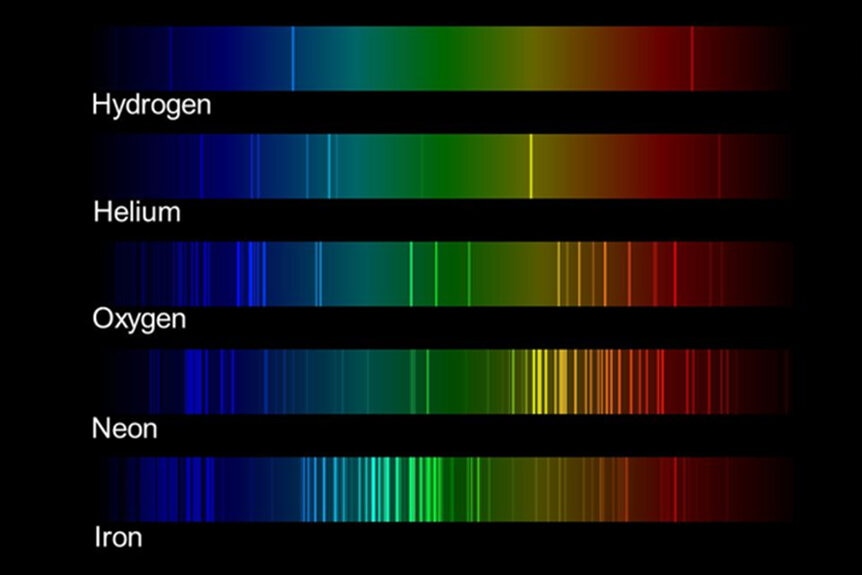
Smith’s work relied on spectroscopy, a way of analyzing the properties of a material by way of its chemical structure. When a material is energized, either by a star or by some other source, it will re-emit light. That’s true even of you, that’s how infrared goggles work. They’re picking up on the non-visible light coming off of your body. We think of it as heat. The spectrum of that light depends on a number of things including temperature and what the material is made of. See, each element shines a little bit differently, emitting light in different parts of the spectrum. By carefully measuring the light scientists can learn a lot about the thing they are measuring.
A geologist, for instance, might put a strange piece of rock into a spectrometer to see what it’s made of. Usually, that involves blasting a small piece of the rock with a powerful laser and measuring the light coming off the vapor. Astronomers also use spectroscopy to learn about the composition of distant objects. We can’t scoop up a piece of an exoplanet millions of light years away, but we can measure its light. The spectral signature coming off a planet gives astronomers a clue as to what its atmosphere or surface materials are made of, even from millions of light years away.
Smith used spectroscopic measurements of each element from the periodic table and converted them into sound. That, in and of itself, isn’t new. Scientists have created acoustic representations of the elements before, but never with this level of detail. Some elements, particularly the ones on the heavy end of the table, have a lot of information making their signatures noisy. Previous works have focused only on the brightest wavelengths, assigning them to individual notes. It’s sort of like turning the volume on your favorite song all the way down. It might be easier for you to transcribe but you’re only capturing the loudest parts.
Smith wanted an acoustic representation which preserved as much data as possible. To achieve that, he created software to automatically convert the spectroscopic data to sound. It took the wavelengths and gave them sine waves depending on frequency and brightness. As is often the case when converting natural phenomena (like the aforementioned black holes) the frequencies involved are well outside what the human ear can hear. So, Smith scaled the audio down until it sat within the audible range.
The result is a chemical keyboard which becomes increasingly chaotic the further along the scale you go. Light elements like hydrogen or helium are recognizable as collections of notes, something akin to a cord. As you approach the heavier elements, however, some become discordant as hundreds or thousands of frequencies blend together. Others sound like resounding choirs singing in harmony. If you’re planning to use acoustic chemistry to write the next hit song, there are some elements you’ll probably want to avoid. Smith endeavored to stay true to the data, even if it wasn’t pretty, which means some elements don’t sound particularly good.
What is perhaps more exciting than the songwriting potential, at least for scientists, is a novel way of investigating chemistry. Looking at spectra on paper, the lines can blend together, making it difficult to distinguish between them. Music, however, generates a more intuitive if visceral response. A person might be able to tell the difference between the sounds of certain elements, even if they can’t articulate why. Future scientists might develop acoustic tools for analyzing spectroscopic data from distant planets, potentially streamlining our hunt for interesting worlds. If we do one day discover alien life or even a habitable planet around another star, it might be because we heard them before we saw them.
If individual atoms can sing, why not animals? Catch Sing and Sing 2 (the sing along edition), available from Universal Pictures.