Create a free profile to get unlimited access to exclusive videos, sweepstakes, and more!
Asteroid Collisions Create a Forbidden Crystal

Crystals are pretty. They’re also pretty interesting. They’re found in nature in stunning variety, including all kinds of bizarre shapes. I find a lot of these shapes pleasing aesthetically due to their symmetry. Some are box-shaped, some hexagonal … but they’re all fascinating.
Crystals get this symmetry because of the way atoms interact. They’re like puzzle pieces, connecting only in certain ways. For example, carbon atoms can bond to each other to form sheets that are a single atom thick, but contain zillions of carbon atoms interconnected as hexagons. We call this graphene. But they can also connect to form tetrahedrons, four-faced triangular pyramids. The property of that crystal is very different, so we give it a different name: diamond.
Over the years crystallographers have found that there are four kinds of symmetries natural crystals can have: twofold, threefold, fourfold, and sixfold. These are all based on taking a shape and rotating it 360°. For example, take an equilateral triangle. If you spin it 360° it looks the same. But it also looks the same if you spin it 120° and 240°. So after spinning it all the way around, you get the same pattern three times: a threefold symmetry.
A regular hexagon has six sides, and looks the same after you spin it 60°, 120°, 180°, 240°, 300°, and finally 360°. So it has sixfold symmetry.
Now, you could theoretically have a fivefold symmetry, for an object that goes through multiples of 72° rotations (after five of those you’re back to 360°). But that’s never found in nature. The other symmetries are very strong, and crystals find themselves displaying those instead.
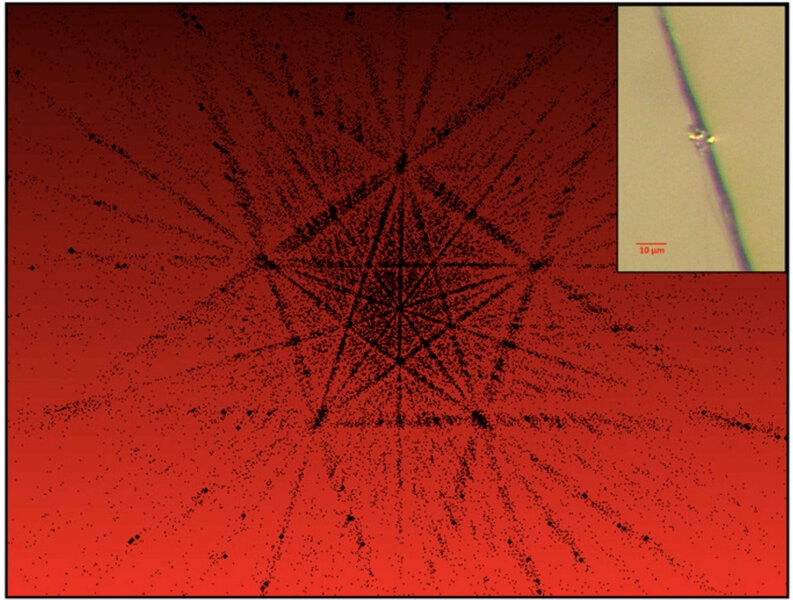
… until recently. In the 1980s scientists were able to create a fivefold crystal in the lab, which they called a quasicrystal. It’s tough to do, but it can be done. Still, the big question remained: Can that be found in nature?
The answer we now know is: Yes! Scientists found two such crystals in nature, and they came from the same source: a meteorite that fell in far eastern Russia called the Khatyrka meteorite.
Meteorites generally come from asteroids. Collisions break them up into smaller pieces, and sometimes these fall to Earth. Khatyrka has an unusual composition, and when examined microscopically indeed shows signs that it had undergone collisions while it was still part of its parent asteroid body. The scientists wondered if they could replicate this. They created a series of disks made of the same minerals found in the meteorite and stacked them like coins, making something like a hockey puck. They then used a large gas gun to blast it with a projectile moving at about one kilometer per second, which is a typical (or perhaps somewhat slower) collision speed for asteroids in space.
When they examined the result, they found a quasicrystal with fivefold symmetry, which is now named icosahedrite. The chemical formula for it is Al63Cu24Fe13: 63 atoms of aluminum, 24 of copper, and 13 of iron. No wonder it’s so hard to find it in nature!
It’s not entirely clear in detail how it formed, though sudden compression, heating, and then cooling play a role. On Earth those conditions are very rare, but they’re more common in asteroids. The weird composition of the meteorite plays some part too, having the right combination of minerals to start with such that in the end the quasicrystal is created.
At this point you may be wondering, so what? I have a few whats for you. One is that nature is more clever than we are. These crystals were once thought impossible, but here they are. They’re rare, but not impossible. Just very unlikely and need very special conditions to form.
Second, this gives scientists more insight into the literal structure of nature. Perhaps quasicrystals will never have a practical application, but even if they don’t they still help us understand how the world is put together.
And third, this hints at new branches in the science of crystallography. What other crystals exist, what other strange compounds? What uses will these have? Maybe none, at least not in our ability to exploit them for technological advances, say (the way silicon was used to make computer chips as an example). But again, the more we understand the rules governing the Universe, the better we understand it, and that is a goal unto itself.
And hey, if we can figure out how to make transporters or warp drives or light sabers, then all the better. But in the meantime, just gaining knowledge is pretty cool, too.
P.S. I’ve been meaning to write about this topic for a little while, but tonight I’m giving a talk about science outreach to members of the American Crystallographic Association at their meeting in Denver, so I thought the time was right.