Create a free profile to get unlimited access to exclusive videos, sweepstakes, and more!
Did life here begin ... out there? Maybe its precursors did.

A brief logical treatise: Life exists on Earth now. Life as we know it is based on chemistry using complex carbon-based molecules (what are called organic molecules) which are built up from less complex molecules (usually called prebiotic molecules). When Earth formed (4.56 billion years ago), it was far too hot for those chemicals to exist. Therefore, at some point after it formed and cooled sufficiently, those chemicals could be found on Earth.
OK, fair enough. So ... where did they come from? Certainly, the early Earth had everything it needed to make these molecules: carbon, hydrogen, oxygen, nitrogen and other trace elements were all over the place, and the conditions to form the molecules from these elements probably existed as well.
But that doesn’t mean they formed here! What if they came from space?
That may sound silly, but it’s not impossible. Comets, for example, were once thought to be just lumps of ice and rock left over from the formation of the solar system. We now know they have a far more complicated history, and they are also space-based chemistry sets, creating all sorts of complex prebiotic materials on their surfaces. We also know they impact the Earth and can bring that material here. There’s conflicting evidence whether Earth’s water came from comets, but they could have easily dropped megatons of prebiotic goo on us a few billion years ago.
More recent observations have made this issue even more interesting. Prebiotic material has been seen around newly forming stars; that is, clouds of gas and dust in the process of condensing into stars. And astronomers have just announced they found yet another molecule: methyl isocyanate (meth-uhl EYE-so-SIGH-en-ate, if you want to impress people at parties with your elocution). It’s a simple molecule with the formula CH3NCO, and is part of the biological process to create peptides, protein-like molecules that have a wide variety of uses in biology*.
Mind you, we've seen other prebiotic materials in space: sugars, for example, and alcohol. Even methyl isocyanate has been seen before in forming stars, but those were massive stars, and conditions there are different than for stars more like the Sun. In this case, astronomers using the Atacama Large Millimeter Array (ALMA) observatory detected the prebiotic molecule in a pair of forming stars called IRAS 16293-2422, located about 400 light-years from us. These stars orbit each other in a binary system, and each has a mass roughly half that of the Sun. The conditions under which they’re forming may be very similar to those in which our own Sun and planets formed. The stars in IRAS 16293 are deeply embedded in a thick cloud of gas, dust and various molecules, and the cloud is very cold, just a few degrees about absolute zero, and only warms up near where stars are forming.
Still, a lot of chemistry can go on in these clouds, especially near the protostars. Temperatures change, pressures change, shock waves slam through the material, and all these things can spur interesting chemical reactions ... including the creation of methyl isocyanate.
As an aside, the astronomy I have done is studying gas that’s hot. When you energize the atoms, the electrons in them jump up to higher energy levels. When they jump back down, the electrons give off a very specific color of light, different for each energy level and each atom emitting it. By measuring the colors of light emitted by the gas you can, among other things, determine its composition.
Cold gas can be different. While you still get those kinds of emissions, another kind is important, too. Some molecules can absorb energy that makes them physically spin around an axis like teeny tops. They then emit that energy back into space by giving off very long wavelength light, like the kind radio telescopes or ALMA can detect. It too acts like a fingerprint; the wavelength tells you what molecule you’re detecting.
This is how the astronomers using ALMA were able to know they found methyl isocyanate. They were also able to determine it came from a temperature region of about -170° Celsius. Interestingly, their data also indicated the molecule was found both on icy grains as well as in a gaseous state, so it may have multiple origins.
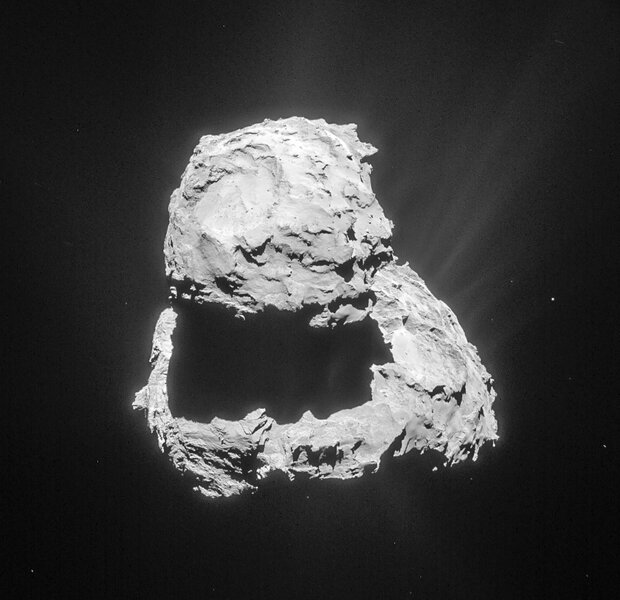
Now, here’s the fun bit. Remember the comet 67/P Churyumov-Gerasimenko, the one the Rosetta spacecraft orbited for years, returning amazing images and data? It turns out the spacecraft detected what looks very much like methyl isocyanate on the comet, as well. Did this stuff form on the comet’s surface, or was it primordial, dating back to when the comet, itself, formed from the cloud of gas and dust that the Sun and planets did?
Interesting question, isn’t it? We know life on Earth must have formed after it cooled, and we know it started up as prebiotic material underwent chemical reactions, combining to form more complicated stuff over the eons, until self-replicating molecules appeared. After that, well, life found a way.
But we’re still not sure where those prebiotic materials came from. Maybe they formed here. Maybe they formed on comets. Maybe the comets, themselves, got them from even earlier, when the solar system, itself, was forming.
Maybe (and this is the way I lean) it’s a combination of all these things. But we’re getting closer all the time to figuring that out. Maybe we’ll never know exactly, but we’ll find clues that tell us the possibilities. That’s wonderful.
Let me take a step back and comment on how wonderful this all is. For thousands of years we could only speculate, mythologize, on how we came to be. Now, with science, we can actually figure it out. And it doesn’t just come from biology, or even chemistry, but astronomy, planetary science, physics, engineering, and more. You need all these fields, working together, to understand the puzzle that is the Universe. The cool thing about objective truth is that, for it to be true and truly objective, all the evidence must agree about it. If something is real, then all aspects of science should agree on its existence.
When it comes to the origin of life, we’re on that path. We’re using the full arsenal, the full capabilities, of science to work on the answer.
I strongly think — maybe not today, maybe not even in a decade, but eventually — we’ll figure that out. When used honestly and rigorously, that’s what science does.
*Methyl isocyanate is also very toxic, but is useful in creating adhesives and rubber and other substances. Chemical companies use it large quantities of it, usually safely. However, in 1984, a large quantity leaked from a Union Carbide India Limited pesticide plant, killing over 3700 people and injuring over a half million more. It’s easy, even fun, to talk about these chemicals in isolation, or their implications for the origin of life. But I think it’s proper and respectful to keep in mind their less savory qualities.