Create a free profile to get unlimited access to exclusive videos, sweepstakes, and more!
Is deadly poison the secret to the emergence of life on Earth and elsewhere?
Cyanide turns out to be the ultimate in acquired allergies.

Generally speaking, it’s a good idea to stay a long way away from cyanide. A couple hundred milligrams, administered orally, is fatal within hours. Ingestion, either through digestion or respiration, brings on symptoms including confusion, vomiting, and injury to the heart and lungs. Even those who survive can have lifelong debilitating disease as a result. In short, it’s super bad stuff and doesn’t play well with biology. Which is why it’s so weird that it might have been an essential ingredient in the formation of life on Earth.
A recent paper by Ramanarayanan Krishnamurthy from the Department of Chemistry at the Scripps Research Institute, and colleagues, reveals that in addition to just absolutely murdering living things, cyanide is an excellent metabolic pathway for prebiotic chemistry. The results of the team’s research were published in the journal Nature Chemistry.
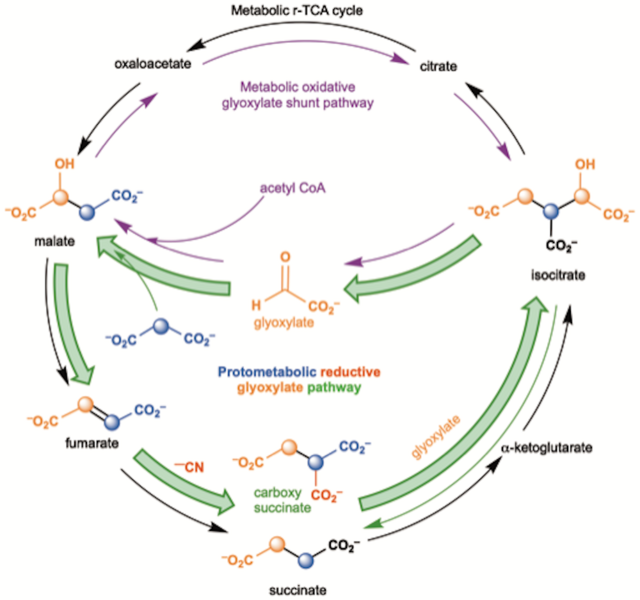
“Cyanide is one of the molecules which we think are a part of early atmospheres, including Earth’s,” Krishnamurthy told SYFY WIRE. “It can lead to the formation of amino acids and give rise to other building blocks.”
The team was mostly interested in the way cyanide interacts with keto acids and the sorts of chemical reductions which take place in the presence of the poison. Prior research had primarily looked at metals as a potential catalyst for early Earth biology. Metals are able to donate electrons and create the sorts of reactions we see in modern life on our planet, but Krishnamurthy and team thought that might be putting the cart before the horse.
“Biology uses metals in a very specialized context of an enzyme which is able to control the donation of electrons,” Krishnamurthy said.
Without the benefit of those enzymes, the electrons in metal interactions act as free radicals and wreak destruction on biomolecules. The question then was how early biology got started, using the sorts of interactions we see in life today, without those enzymatic protections already in place. Using current biology as the framework for how things got started left us with a lot of unanswered questions, and this new paper suggests the answer might have been cyanide.
“Cyanide is a much more benign reagent which reacts with these molecules, transforms them, but does not decompose them. It actually even prevents the decomposition,” Krishnamurthy said.
To be clear, the question of exactly how life arose on Earth has not been answered. But the knowledge that cyanide is capable of performing the same function as other reagents in the sort of chemical soup which is believed to have been present on the Earth shortly after formation, adds a piece to the growing puzzle.
These sorts of cyanide reactions have possible implications not just for life on the early Earth, but also to how we go about searching for potential signs of life on other planets.
“When we analyze meteorites that come from different sources or we look at the atmosphere of an exoplanet, we’re looking for signs of different molecules which might suggest biological processes,” Krishnamurthy said. “On meteorites, we find some amino acids and the question is where did they come from?”
Strangely enough, this new chemical model offers two potential interpretations which are almost at odds with one another. One way to interpret the model is that molecules we previously associated to biological processes might arise abiotically through ordinary chemical interactions. If that’s the case, it might make finding signs of life elsewhere more difficult. The other way to interpret it is that these cyanide-based interactions could suggest the presence of a wholly different form of life.
“If there was early life which used cyanide, it could completely bypass some problematic molecules and offer a reductive cycle which is simpler and easier to operate. Therefore, it suggests if there were earlier lifeforms that operated through this simple chemistry, there could be another kind of life we haven’t yet contemplated,” Krishnamurthy said.
Either there are more chemical compounds suggestive of biology, or there are fewer. Which interpretation is the correct one is not yet clear. One way forward is to answer the question of what happens after these simpler cyanide interactions. Is there a path between these types of interactions and the ones we see happening on Earth today? It’s possible, and it wouldn’t be the first time life changed to use chemical interactions it didn’t use before.
“On the early Earth we didn’t have a lot of oxygen and early life was not using oxygen. Then, 2.5 billion years ago when oxygen arose, life began to use it. This kind of switchover of building blocks has happened and it could have happened earlier when cyanide was an abundant source for chemistry,” Krishnamurthy said.
Here’s hoping the paradigm shift holds up and cyanide chemistry is shown to evolve into what we’re familiar with today. It would go a long way toward answering once and for all how life emerged on our planet. Also, we’re not quite ready to endure the idea of aliens with literal poison in their every cell. Our first contact could be our last.