Create a free profile to get unlimited access to exclusive videos, sweepstakes, and more!
Captain Planet approved! Plant-based plastics rapidly break down into fertilizer
The power is yours!

The development of plastics allowed for a revolution in commercial, industrial, and household items. Take a look around you and you’re almost guaranteed to find plastics within eyeshot. As a result of their convenience, many things which were once designed to be robust and reusable have been replaced by disposable single-use plastic items.
Today, we produce about 330 million tons of plastic each year. According to the EPA, only about 9% of that is recycled, leaving hundreds of tons of waste piling up in landfills, on roadsides, and in the oceans.
One strategy for combating the climate effects of producing so much plastic is shifting from oil-based materials to bioplastics made from plants. While these plant-based products are better for the environment than their petroleum-based counterparts, they still create waste, and making the switch won’t solve the problem of plastic waste on its own. What we need are robust systems for recycling bioplastics in an environmentally friendly way.
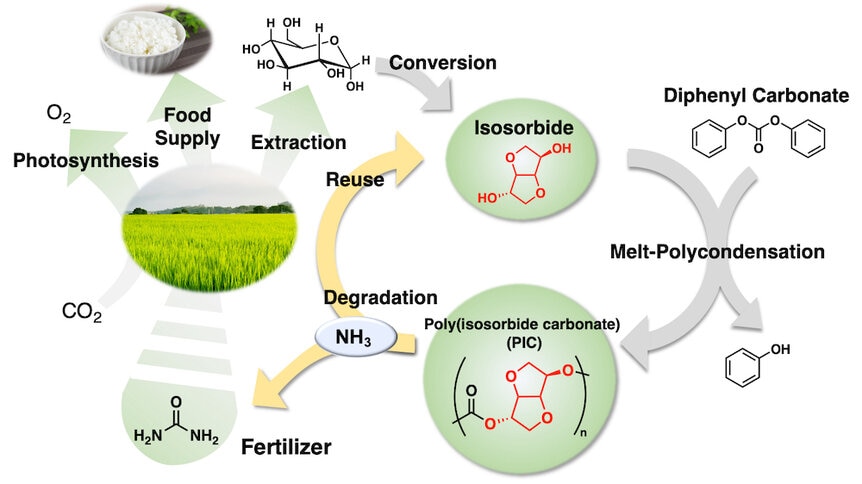
New research by Daisuke Aoki at the Department of Chemical Science and Engineering at the Tokyo Institute of Technology and colleagues demonstrates a process by which bioplastics can be broken down into an efficient fertilizer for growing plants. Their results were published in the journal Green Chemistry. The process turns bioplastics into fertilizer, which can be used to grow plants for food or to make more plastics, the whole process is circular.
To prove their concept, Aoki and team used polyisosorbide carbonate (PIC) materials and an aqueous ammonia solution, both materials that are already readily available on the market. The structure of PIC plastics are pretty simple. Essentially you have a polycarbonate chain of isosorbide connected by carbonate links. Breaking them down is simply a matter of destroying the links between molecules. It’s important to note that some bioplastics may have other materials added in depending on the manufacturing process, which could impact what they break down into, but suitable bioplastics can be made solely from isosorbide carbonate chains.
“The majority of bioplastic available on the market is made of isosorbide,” Aoki told SYFY WIRE. “When you mix it in ammonia solution and add heat, the plastic breaks down. The linkages are decomposed by the ammonia and become urea.”
Placing the plastic in an ammonia solution and heating to 90 degrees Celsius (194 degrees Fahrenheit) breaks it down to its component parts in about 6 hours. Even at 30 degrees C (about 85 degrees F), a little warmer than room temperature, the plastic will break down in about 24 hours, according to Aoki. What you’re left with is just isosorbide and urea, ingredients which make for good fertilizer, without any biproducts in need of disposal. In fact, the ratio of isosorbide and urea leftover after the decomposition of PIC materials appears to be ideal for plant growth, showing a marked increase in weight compared to other interventions.
“We did some plant growth experiments with a model plant A. thaliana, and measured plant growth by calculating the weight of the leaves,” Aoki said. “Using only isosorbide, there was a small increase in growth but almost the same as doing nothing. Using only urea, we get an increase in weight. The final condition was a mixture of isosorbide and urea and we showed great effect on the growth of the plant.”
The researchers showed that bioplastics could be recycled effectively and inexpensively using materials which are already widely available, and without the need for vast amounts of energy. Aside from ammonia solution, the only needed component is heat, which is already used in the incineration of plastics today.
Moreover, according to Aoki, the whole process is carbon negative, with the added benefit of replacing or supplementing existing fertilizers.
“The process is scalable. It’s a simple reaction and only requires the right amount of ammonia solution for what you want to decompose. The ratio of ammonia needed is relatively low and you can get the solution from a pharmacy. A child could do this experiment with the right knowledge,” Aoki said. “The key is that all the ingredients be non-toxic. Plastics, even bioplastics, which contain toxic components will result in toxic monomers along with the urea from the carbonate link breakdown.”
Modifying our economic structures in order to combat the effects of climate change is often seen as a herculean task with costs outweighing any potential benefits. This research shows that change is possible with already existing materials and relatively low energy or monetary costs. All it will take is a little out of the box thinking and the willingness to change.