Create a free profile to get unlimited access to exclusive videos, sweepstakes, and more!
Radioactive plutonium from a nearby supernova found on Earth

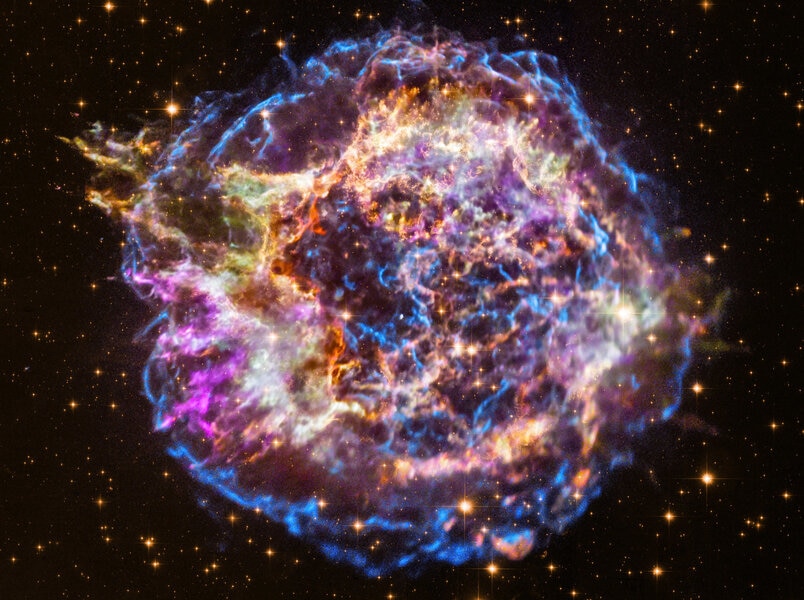
Two and a half million years ago, a cosmic catastrophe unfolded not far from Earth.
In a cluster of young stars just over a hundred light years away, a particularly massive star ran out of fuel. It had gone through its supply of hydrogen, helium, carbon, even oxygen, neon, and silicon. Its core was iron, and that cannot be fused to make energy. The core collapsed, generating a wave of energy so colossal it ripped through the octillions of tons of outer layers of the star, ripping it apart, and creating a supernova.
An exploding star this close to Earth has consequences. It possibly caused a mass extinction on Earth, ending the Pliocene epoch and ushering in the Pleistocene, killing off a large amount of marine life (though to be fair, the connection between the two events isn’t solidly confirmed).
But there was more. A millennium or so later, a wave of matter blasted out by the dying star at a fraction of the speed of light had crossed the depths of interstellar space, sweeping over the Earth, and depositing elements created in the forge of the supernova itself onto our planet.
Some 2.6 million years later, we can still detect these elements. Sediments brought up from the ocean floor and dated to that time period show elevated amounts of iron-60, a radioactive element with a half-life, coincidentally, of about 2.6 million years. The only known source of iron-60 is in a supernova, and with such a short half-life any of this material created with the Earth itself billions of years ago is long gone*. It is therefore, in many ways, the smoking gun of a nearby supernova.
Other elements have been found at elevated levels in this sediment as well, including manganese, which again we know is made in a supernova. Controversial at first, the idea that a star exploded nearby not long ago geologically or astronomically speaking is accepted truth now.
And now new research has found yet another fingerprint of this long-dead massive star buried in our ocean’s floor: plutonium-244, a radioactive element with a half-life of about 80 million years. While again this is yet more evidence of the nature of the catastrophe that befell the Earth, it also has interesting astrophysical implications.
Plutonium is what’s called an r-process element. The “r” stands for rapid, meaning it’s created quickly in extremely dense, hot environments, like the blast wave from a supernova as it eats its way out of the interior of the star. Elements can capture neutrons in their nuclei, and through various additional processes this turns them into heavier elements. Neutrons are in abundance in the supernova, so heavy elements like plutonium can be created in copious quantities.
It’s much more rare than iron, though. While several million iron-60 atoms per square centimeter were found in the ocean sediments dated to 2.6 million years ago, only a few dozen plutonium-244 atoms were found in the same layer.
First of all, let me say how amazing this is in the first place. The upper layers of sediment are polluted with plutonium from nuclear weapons tests starting in the 1950s. They found quite a bit of that in the upper sediment, and some of it mixes down to lower layers, so that needs to be accounted for. But still, seeing plutonium at all is incredible. That they were able to count a few dozen to a few hundred atoms of it per square centimeter is a testament to the scientific method and equipment developed for this. Mind you, a cubic centimeter of sediment has well over 1025 molecules in it, so finding a few hundred atoms of plutonium is remarkable.
But more than just finding any, the amount they found is interesting. It’s possible that a supernova can generate the ration of iron to plutonium seen, it doesn’t match the ratio seen in space. Other studies indicate the amount of plutonium should be higher, which in turn means there must be another source of it in space.
This gets complicated rather quickly, but note that plutonium-244 has a longer half-life than iron-60. That means it could’ve been made by some other process long before the supernova blast wave that carried it here occurred.
The r-process can happen in supernovae, but it is far, far more efficient in more rare events like when neutron stars collide. These superdense objects are what’s left of a massive star’s core after the outer layers explode, and sometimes they can be found in binary pairs. Over billions of years they spiral together, collide, and explode. This creates an incredibly neutron-rich environment, and is likely the major source of r-process elements like plutonium in the galaxy. While rare, they make so much they dominate production.
It’s possible a close-by neutron-star merger seeded the material between the stars near Earth with r-process elements many million of years ago. When our neighboring unnamed star exploded just 2.6 million years ago, the huge blast swept up this already-created plutonium-enriched matter and deposited it on Earth along with the iron-60 made in the supernova.
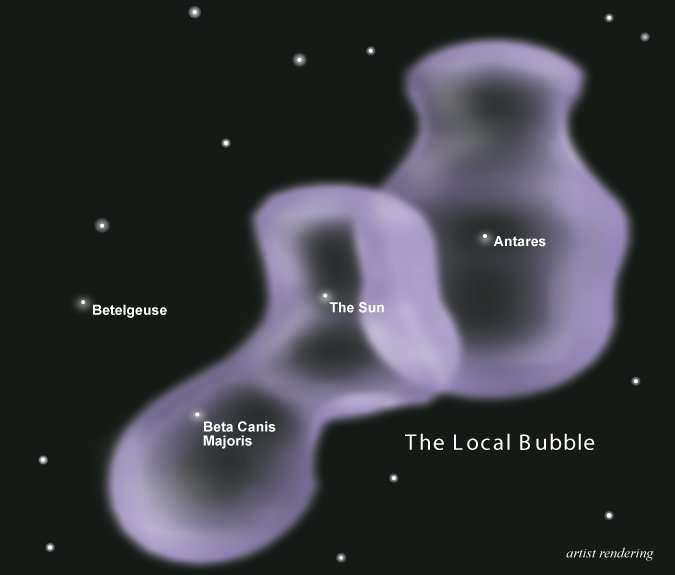
Like I said, it’s complicated. And there’s more! There’s decent evidence that more than one supernova went off in the past 7 million years, too, possibly two or as many as four. The Sun exists in a huge cavity hundreds of light years across called the Local Bubble, which was almost certainly carved by the winds of material sent out by exploding stars nearby. The likely culprit is a clutch of several hundred young stars called the Scorpius-Centaurus Association, which lies a few hundred light years from us. It has lots of massive stars that will explode someday, and certainly some had many millions of years ago, too.
It’s inevitable we’ll see more supernovae from this group in the next few million years. Since the last few, the association has moved away from us and presents less of a danger, so a mass extinction is less probable (though I’d be happier if they can hold off for another few hundred thousand years at least, which is likely). But if one were to go off, it would be a boon to astronomers. Any relatively nearby (but not too nearby) supernova is easier to study, but with one from Sco-Cen we might be able to determine what effects this may have had on our planet all those millions of years ago, and also better understand our own connection with the cosmos.
In this case, our literal direct connection.
*The half-life is the time it takes for half the amount of a sample of an element to radioactively decay to some daughter element. Start with a million atoms, and in one half-life 500,000 will be gone. Another half-life later and you’re down to 250,000 remaining, then 125,000, etc. Statistically, this can be used to determine ages of samples, though in detail the process is complicated.